What causes dementia?
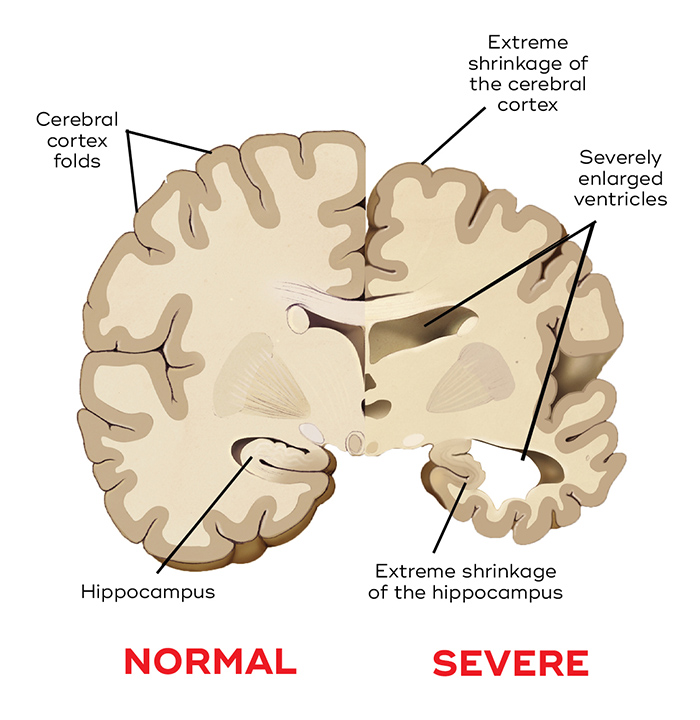
Dementia is caused by neurodegeneration – the damage and death of the brain’s neurons. Depending on the types of neurons and brain regions affected, the form of dementia differs. For instance, frontotemporal dementia mainly affects the frontal and temporal lobes, whereas Lewy body dementia affects part of the frontal lobe and the motor cortex. In the brains of patients with advanced Alzheimer’s, there is widespread degeneration, and damage to the hippocampus – a part of the brain essential to memory formation, and which produces new neurons. The loss of brain tissue results in a shrunken brain, enlarged ventricles and more space between the folds.
Most disorders associated with dementia are progressive, degenerative and irreversible. Some causes are treatable, including head injury, brain tumours, infections (e.g. meningitis), hormone and metabolic disorders, hypoxia, drug abuse and alcoholism.
Toxic aggregates
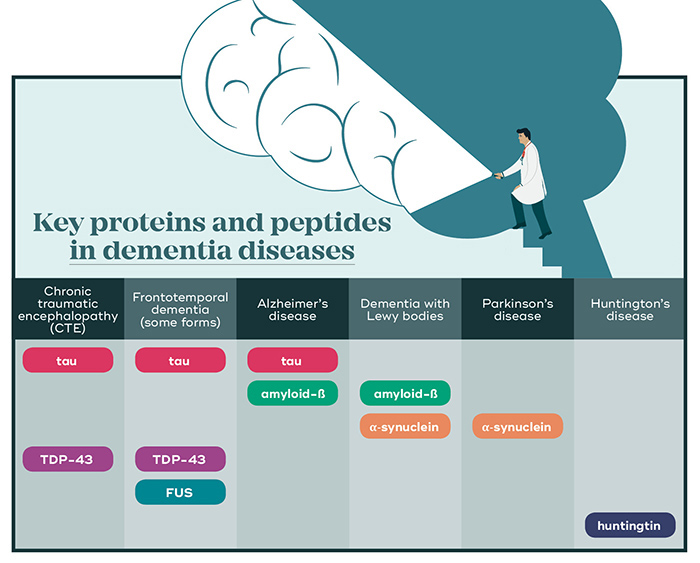
In most dementias, build-up of toxic proteins is a key part of brain degeneration.
This causes a loss of the contact points between neurons (known as synapses) as well as a loss of the neurons themselves. What sparks this neurodegeneration remains unknown, but for dementias other than vascular dementia, a build-up of toxic proteins and the loss of their normal function are defining characteristics.
Proteins can naturally aggregate as the body’s systems for clearing them start to decline, which occurs increasingly as we age. In neurodegenerative disease, these toxic clumps, known as aggregates, can damage or kill neurons.
In Alzheimer’s disease, two key players called amyloid-ß (a peptide that forms plaques around neurons) and tau (a protein that forms ‘tangles’ inside neurons) are at play. In dementia with Lewy bodies (which also forms amyloid-ß deposits) and Parkinson’s disease, the major aggregates are formed by the protein α-synuclein. In frontotemporal dementias, deposits of TDP-43, tau or FUS protein are found. Each type of protein aggregate eventually leads to the death of affected neurons.
A problem called amyloid-β
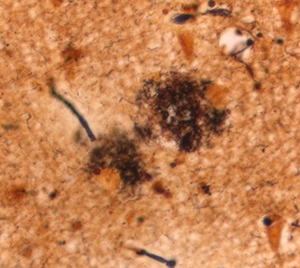 The main component of the hallmark plaques seen as lesions in the brains of Alzheimer’s patients (left) is formed by a peptide called amyloid-ß (beta). Certain neurons, particularly in the cortex and hippocampus, create amyloid-ß. Its function is not well understood, but it has a role in neurogenesis (the creation of neurons), memory, and the normal operation of message transfer betwen neurons. When too much is made, or too little cleared, clumps of amyloid-ß build up around and between neurons. As these plaques grow in size, they envelop and destroy the dendrites (branches) of neurons, interfering with their ability to communicate.
The main component of the hallmark plaques seen as lesions in the brains of Alzheimer’s patients (left) is formed by a peptide called amyloid-ß (beta). Certain neurons, particularly in the cortex and hippocampus, create amyloid-ß. Its function is not well understood, but it has a role in neurogenesis (the creation of neurons), memory, and the normal operation of message transfer betwen neurons. When too much is made, or too little cleared, clumps of amyloid-ß build up around and between neurons. As these plaques grow in size, they envelop and destroy the dendrites (branches) of neurons, interfering with their ability to communicate.
The role of tau
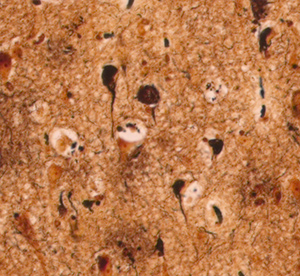 Tau is a protein that normally has an important role in maintaining the structure of a neuron’s axon (the long cable that transmits signals). In dementias such as Alzheimer’s and frontotemporal dementia, more tau is made, eventually accumulating in the cell body and dendrites. Here, it forms large deposits known as ‘neurofibrillary tangles’ – clumps that build up and gradually interfere with the neuron’s function and eventually kill it.
Tau is a protein that normally has an important role in maintaining the structure of a neuron’s axon (the long cable that transmits signals). In dementias such as Alzheimer’s and frontotemporal dementia, more tau is made, eventually accumulating in the cell body and dendrites. Here, it forms large deposits known as ‘neurofibrillary tangles’ – clumps that build up and gradually interfere with the neuron’s function and eventually kill it.
Why do protein deposits form in the first place?
Researchers want to know why these protein aggregates form in the first place, how they distribute in the brain, how their signalling works, and how they and their precursors cause neurodegeneration.
A range of theories have been put forward to explain what kickstarts damaging protein aggregation. In the case of Alzheimer’s, this includes problems with the way oxygen is metabolised in brain cells and the movement of internal cell contents. The brain’s response to inflammation, and its systems for clearing waste could also play a role.
One main theory is that once amyloid-ß begins to accumulate, it then promotes the build-up of tau. But the relationship is not simple, because tau also has a role in influencing the toxic effects of amyloid-ß.
Although some genetic risk factors for dementias have been identified, particularly for Alzheimer’s disease (see page 12), we still don’t know how these act to influence protein aggregation and cause degeneration. This is a key area of research focus, and knowing the answers to these questions is crucial to the prevention and treatment of dementia.
The role of inflammation
Inflammation has long been thought to be a side effect of dementias such as Alzheimer’s, as the body ramps up its immune system in response to the disease. But recent research has confirmed that inflammation actually contributes to the disease process. One clue was a report that people treated with anti-inflammatory drugs for arthritis had a lower incidence of Alzheimer’s. This has now been backed up by large genetic studies.
Normally, the brain’s inflammatory cells help to prevent damaging build-up of amyloid-ß by clearing it away, and it’s thought that mutations in inflammatory genes hamper this process. Inflammation is a sign of the immune system kicking into gear, and in the initial stages of disease, this is beneficial. However, in the case of Alzheimer’s, when the disease becomes more advanced, chronic inflammation can set in and add to the toxic insult the brain receives. The brain’s immune response to inflammation is therefore believed to play two critical roles in developing Alzheimer’s disease.



