The DNA code in genes inherited from our parents gives instructions for much of the way our physical bodies are made, our risk factors for diseases, our personalities, and individual directions for cells to develop into particular types—neurons, for example.
But our environment and experiences also have an impact on how our bodies and brains look and work. The concept that a gene’s function can be altered by experience—by turning it up or down without changing the underlying DNA code—is called epigenetics.
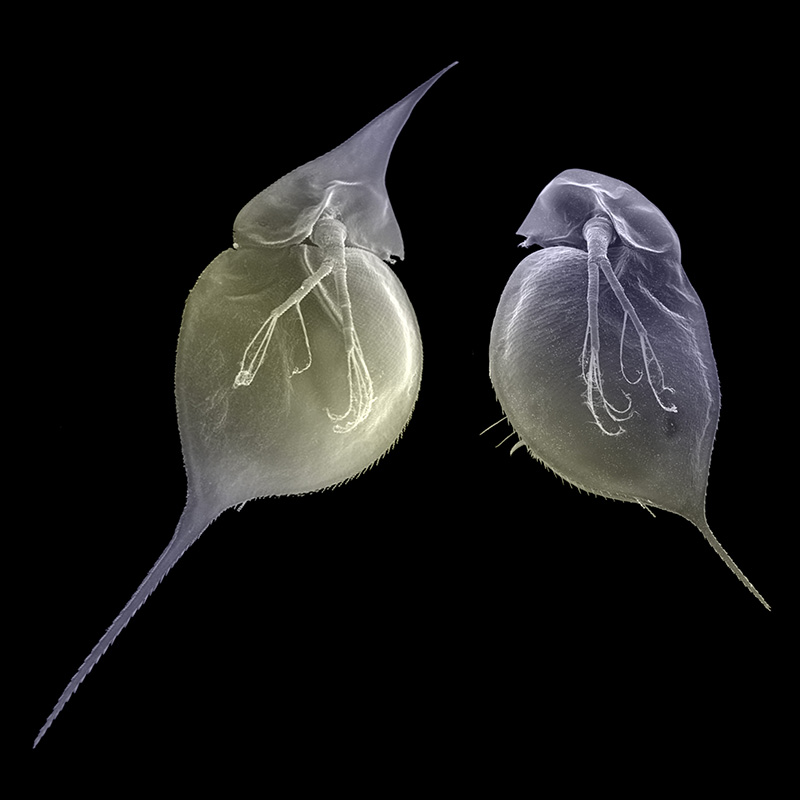
Studies on water fleas have demonstrated this epigenetic effect. These insects grew horns on their heads in response to predatory signals in the water and would pass these features on to their offspring as long as the signal was in their environment.
As soon as the signals were gone, the next generation of offspring were born with normal heads. This is a classic example of an epigenetic change in response to an environment, which doesn’t involve a change in the underlying DNA code.
Professor Oded Rechavi’s lab at Tel Aviv University studies these mechanisms in the roundworm
C. elegans. Their earlier work showed that epigenetic changes in worms can produce adaptations across generations. More recently, they discovered that epigenetic changes in neurons affect the ability of the worm’s offspring and descendants to forage for food.
From simple organisms to the complex human brain
Researchers are now moving from studying epigenetics in simple organisms to looking at the complex human brain. Epigenetics in the brain, in principle, changes gene activity in response to a variety of experiences, including exposure to drugs of abuse or environmental toxins, changes in nutritional factors, and simple daily events such as social contact.
But how is a gene turned up or down after an experience? The answer is by reversibly modifying our DNA. Back in the early 1970s, Russian scientist Professor Boris Vanyushin was studying how learning affects DNA.
He found that an increase in chemical tags, called methyl groups, that bind directly to DNA was related to the strength of memory. This major discovery changed the way we think about the relationship between our DNA and experience.
We now know that, like a dimmer switch, these tags on DNA can turn genes up or down depending on the circumstance. This was a turning point in neuroscience because it revealed that our DNA is not our destiny and implies that our environment can profoundly affect the way our brains function, all the way down to our genes—and these adaptations can be passed on to the next generation.
For the past two decades, Associate Professor Timothy Bredy and his team from UQ’S Queensland Brain Institute have been investigating how epigenetics affects memory. They have shown that all four DNA bases of the genetic code—A, T, G and C—are subject to these chemical tags and can work together to influence the strength of different kinds of memory.
The team has also found underlying ways in which these tags emerge, showing that a large part of our genome doesn’t actively code for proteins that make our neurons function correctly, but is instead a sophisticated surveillance system that allows the environment to influence our genes.
Their work has important implications for understanding how memory works and developing new treatments for fear-related anxiety disorders like PTSD.

