Animal models have been fundamental to advancing our understanding of Alzheimer’s disease, but their value is increasingly being questioned.
University of Queensland researchers at the Queensland Brain Institute Professor Jürgen Götz and Dr Pranesh Padmanabhan argue animal models, particularly mice, are and will continue to be indispensable for Alzheimer’s research, particularly if paired with computational modelling.
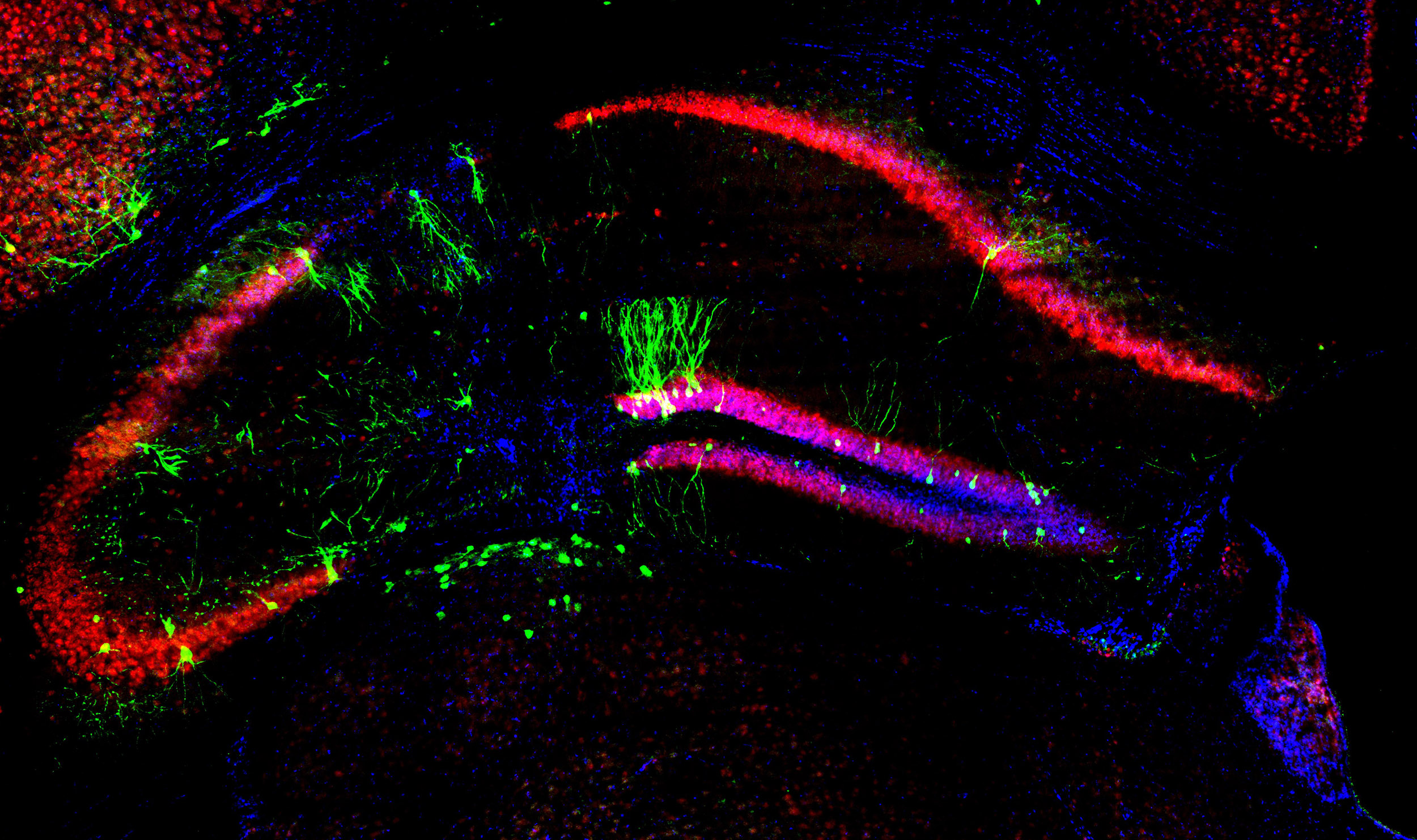
Image by: Ramon Martinez-Marmol
“Despite their limitations, mouse models have contributed to our knowledge of what causes and drives the degenerative process in Alzheimer’s disease and what interventions could potentially slow disease progression,” Professor Götz said.
Despite their clear necessity, and massive effort by researchers with countless clinical trials, the perceived failure of science to deliver a cure for Alzheimer’s disease has often been attributed to a lack of translatability from animal models to humans. Even the real-world effectiveness of two recently approved therapeutic antibodies, aducanumab and lecanemab remains to be determined.
Using a mouse to model a degenerative disease in the human brain is, of course, limited. Mice have much smaller brains and much shorter lifespans than a humans.
Professor Götz said animal models offers distinct advantages in understanding Alzheimer’s in humans, but no animal model can fully replicate the brain function of humans.
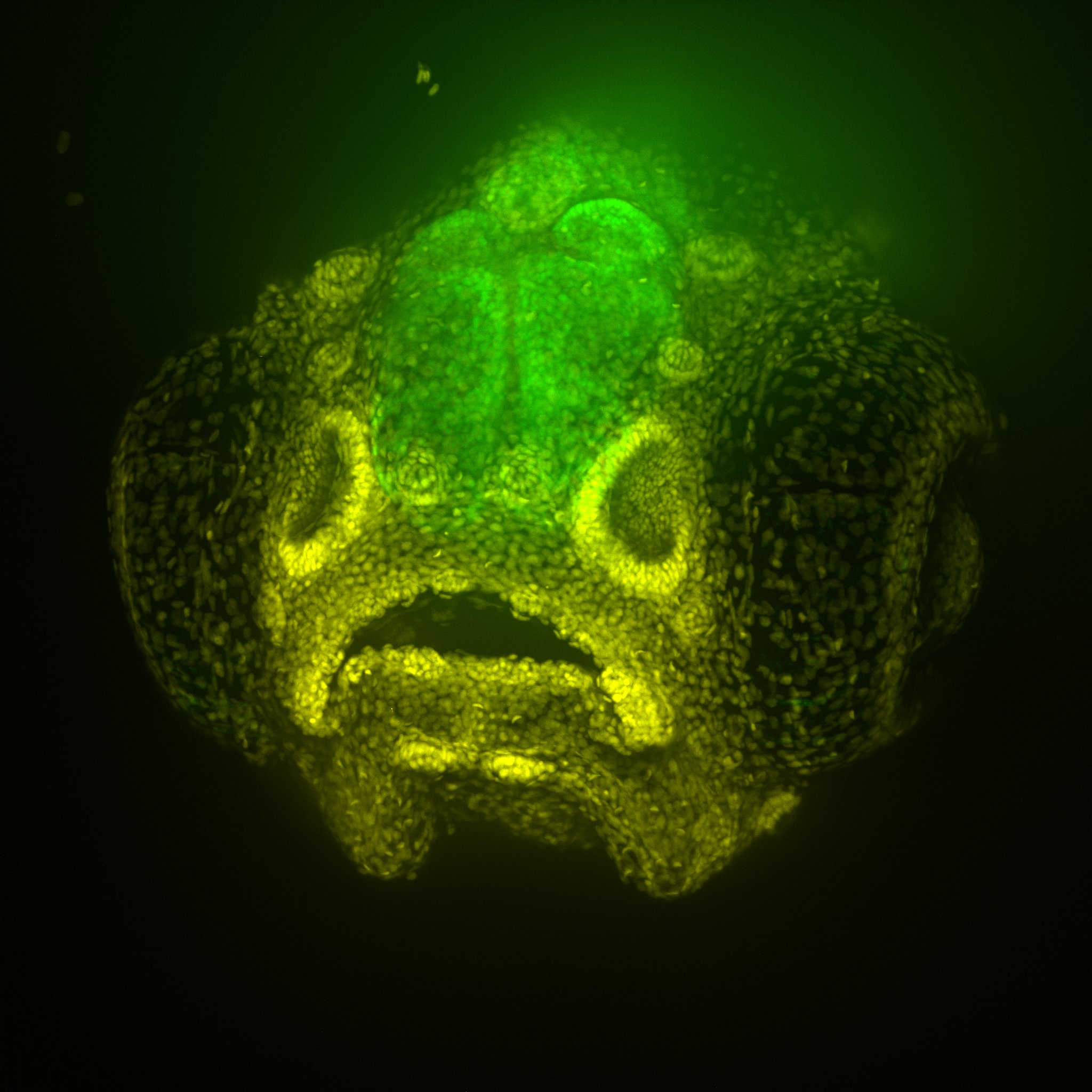
“Larger animals, such as pigs and sheep, which are genetically and anatomically closer to humans, are, in principle, attractive animal models for Alzheimer’s, but they aren’t as easy to study as mice, and the Alzheimer’s pathology is even more difficult to reproduce,” Professor Götz said.
“Some researchers suggest non-human primates may be necessary to model the entire spectrum of Alzheimer’s, but because of ethical considerations, they are not widely used.”
Dr Padmanabhan added that no animal can fully replicate all the functional impairments that characterise Alzheimer’s so researchers can effectively study the systemic picture.
“Existing animal models are useful within the limits of their design, but they don’t capture the entire complexity of human Alzheimer’s disease,” Dr Padmanabhan said.
Brain organoids or “mini brains”, typically generated from stem cells, have emerged as a promising tool for the study of neurodegenerative diseases. But despite being actual human cells, these models come with their own limitations.
Professor Götz and Dr Padmanabhan believe we need a holistic systems-level understanding of how different Alzheimer’s pathologies interact in space and time to initiate and drive the disease.
Dr Padmanabhan explained mathematics could offer a solution.
“Computational modelling has the potential to identify many of the phenomena linked to Alzheimer’s and integrate them into one comprehensive framework to better understand everything that’s going wrong in the brain,” Dr Padmanabhan said.
“By iteratively developing animal and computational models — using mathematical models to validate animal models against human studies and animal models to calibrate mathematical models — we may generate better preclinical tools that improve the translatability of preclinical studies.”
This perspective was published in Nature Aging.



