What worms can teach us about repairing injured neurons

Unlike other parts of your body, such as the skin or bones that grow new tissue to repair damage, nerve cells are not easily replaced. They have to repair themselves, but this process is inefficient, slow and inadequate, if it happens at all. This poses a major hurdle for recovery after injuries to the nervous system, such as traumatic brain injury, spinal cord injury, neurodegenerative disease or stroke. If we are to make advances in repairing spinal injuries, we must first understand the basic mechanisms of how nerve cells repair themselves.
Nerve cells, or neurons, are the basic unit of the nervous system, and billions of them form intricate circuits that are essential for your body’s day-to-day functions. They are unlike any other cells in your body because of their unique cellular structure. A neuron has a long, thin, cable-like structure known as the axon, which transmit electrical messages to target tissues, like muscles. When axons are injured, they can no longer transmit messages and function is affected. The neuron needs to repair that connection to re-establish the line of communication, but this is easier said than done for humans.
To restore function when a neuron’s axon is severed in two, the section attached to the main cell body – known as the ‘proximal’ (near) fragment – needs to successfully regrow and reconnect with its original target tissue. The easiest option would be to reconnect to the ‘distal’ (distant) fragment – the part of the axon separated from the main cell body. But normally after injury, in humans and other mammals, this distal fragment withers and dies, and the proximal axon is left with the difficult - if not impossible - task of regrowing a full length to reach its original target tissue.
However, in many invertebrates such as the sea slug, earthworm, leech, crayfish and the roundworm C. elegans, the esiest option of reconnection the two broken halves of the axon (proximal and distal fragments) does occur; in these species, a repair process known as axonal fusion allows the two fragments of the severed axon to fuse back together, preventing degeneration of the distal fragment and rapidly restoring the neuron’s function. This is much more efficient than regrowing the axon all the way back to original target tissue. So, there is enormous potential for us to learn how these animals spontanously repair their axons and apply our knowledge to promote nerve repair in humans.
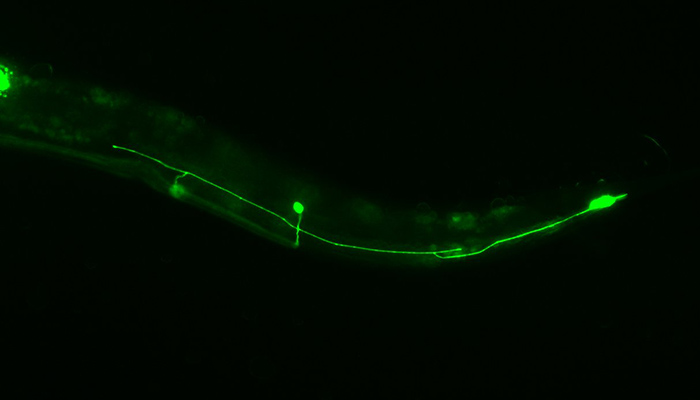
Roundworms (C. elegans) can repair injure axons, by fusing the sections (above) so the whole neuron doesn't have to grow back.
Why use C. elegans as a model system?
Studies in animal models, including C. elegans, flies, zebrafish and rodents, which can easily be genetically modified, allow scientists to have a deeper understanding of the molecules involved in many biological processes, including neuron repair, and how they affect different cells and tissue types within a whole organism.
Sydney Brenner, a 2002 Nobel prize winner, introduced C. elegans as an experimential animal model to study development and neurobiology. Many key discoveries, both in basic biology and medicine, were first made in this 1mm long soil-dwelling nematode worm. C. elegans has only 959 cells and exactly 302 neurons, so its simple biology makes it easier for us to identify the molecules involved in biological processes. At a genetic level, C. elegans shares about 60-80% of the same genes as humans, which means that similar molecules involved in neuron repair are likely to be found in humans.
In recent years, C. elegans has furthered its reputation as a superb model system for research in neuron regeneration, not only for the ease of genetic manipulation but also because it is possible to sever a single axon – something that is difficult to achieve in more complex animals. C. elegans is also semi-transparent, allowing us to use a laser to sever an axon in a live animal and watch in real time as the axon spontaneously repairs itself.
Discovering molecules involved in neuron repair
One way to learn more about the molecular mechanisms of spontaneous repair in C. elegans is to discover the molecules involved in repairing injured neurons. We can do this by selecting worms with mutations in specific genes and testing their ability to repair an axon after laser surgery. If the axon isn’t fully repaired, this suggests that the mutated genes play a role in this process.
Identifying the genes involved in repairing injured axons can help us understand how the proteins encoded by those genes work at the molecular level; that is, how they control the repair process. This is an essential step towards identifying new drugs or approaches that can be used to promote repair of damaged nerves in humans.
Translating knowledge from worms to humans
A study in our lab, published in Nature in 2015, looked at the molecular mechanisms involved in axonal fusion in C. elegans. We found that a neuron responds to injury by changing the composition of the lipid component of its cell membrane, which acts as a ‘save me’ signal. The severed neuron is then repaired by axonal fusion through a protein known as EFF-1.
The question is, can this alternative repair mechanism be activated or artificially induced to repair nerve injuries in vertebrates such as humans? And what other molecules are involved in neuronal repair?
Several large-scale genetic screenings have found hundreds of new molecules that regulate neuron regrowth, the first step of repair. A screening of small molecules also identified potential drugs that could promote regeneration of neurons.
Although these discoveries are rapidly moving the research field ahead, an effective solution to fully repair nerve injuries and neurodegeneration has still not been achieved – but is a highly sought after goal.
As science and medicine have taught us many times in the past, going back to fundamental research and understanding the basic biology of neuron repair might help us finding innovative solutions to problems difficult to solve and translate this knowledge to humans.

