Stereology Overview
Why use it and what can it be used for?
Traditional approaches to counting, such as counting cell profiles, are biased. Furthermore, calculating cell density in sample regions may not reflect a change in cell number or be representative of the whole tissue.
Stereology can be used to accurately estimate the total number of cells within a region of interest by only counting ~200-800 cells. This allows you to make statistically valid quantitative comparisons instead of observing qualitative differences.
It can also be used to determine the size of cells/nucleiwhilst counting, the volume of a region of interest, the length of fibres (e.g. vasculature or neuronal projections), or to analyse three-dimensional cytoarchitecture (e.g. cell-cell spatial relationships).
It is important to understand stereology is time consuming and should NOT be used as a technique to fish for results.
Things to remember:
It takes time.
For each sample group a Pilot Study should be conducted on one animal to determine the best sampling parameters. A pilot study generally takes 1 whole day / region of interest. Following on, additional animals should take ~1/2 a day each.
Identify the region you are counting in.
You need to be able to identify the region you want to count in at all depths throughout the brain. You can use the mouse brain atlas, or sometimes the cells of that region will be clustered and drawing a border around them will be easy. If you can’t accurately identify where you are counting you may need to reconsider your project – varying region outlines can cause big variations in cell counts.
Sample the whole region.
You need to have set of sections that spans right from the beginning of your region of interest to the very end. You do not need every section – often people use serial sections e.g. every 6th section.
Missing sections.
It is OK if a section gets damaged and can’t be plated, or folds up and can’t be imaged – just make a note of it. The important thing is that you need to know that the section is missing so that it can be accounted for in the analysis. Proper stereology will be impossible if there is no record of missing sections.
Sections need to stay thick.
For stereology it is important to have thick sections, for this reason we often cut at 40µm or more. These sections also need to stay thick – if a section shrinks below 20µm thick then it becomes difficult or impossible to carry out stereological analysis.
Discuss your project before you start.
Tissue preparation:
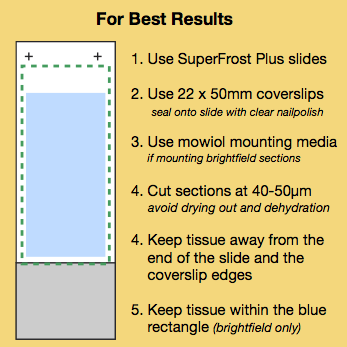
Tissue sections should be cut at 40-50µm.
You can cut thicker but you need to make sure the whole section will be labelled during your staining protocol – often times if the tissue is too thick the stain or antibodies will not reach the middle of the section.
Avoid drying the tissue.
Drying the tissue will make it thinner. Ensure you keep your tissue sections hydrated, avoid processing steps that involve drying, ethanol or xylene, If a section needs to be dried you can rehydrate overnight in PBS+azide.
Mount sections in an aqueous solution.
This is usually done for fluorescent samples but DPX is often used for brightfield. DPX can make sections very thin, especially if xylene has been used in the protocol. For brightfield sections use Mowiol instead of DPX.
For further information about stereology:
Visit the Stereology.info website here.
Visit the MBF StereoInvestigator Website here.
Download the QBI Stereology Handout here.
