This project will investigate how a dietary trace element can regulate ferroptotic cell death to improve cognition in ageing and neurodegeneration.
Objective/mission (the vision)
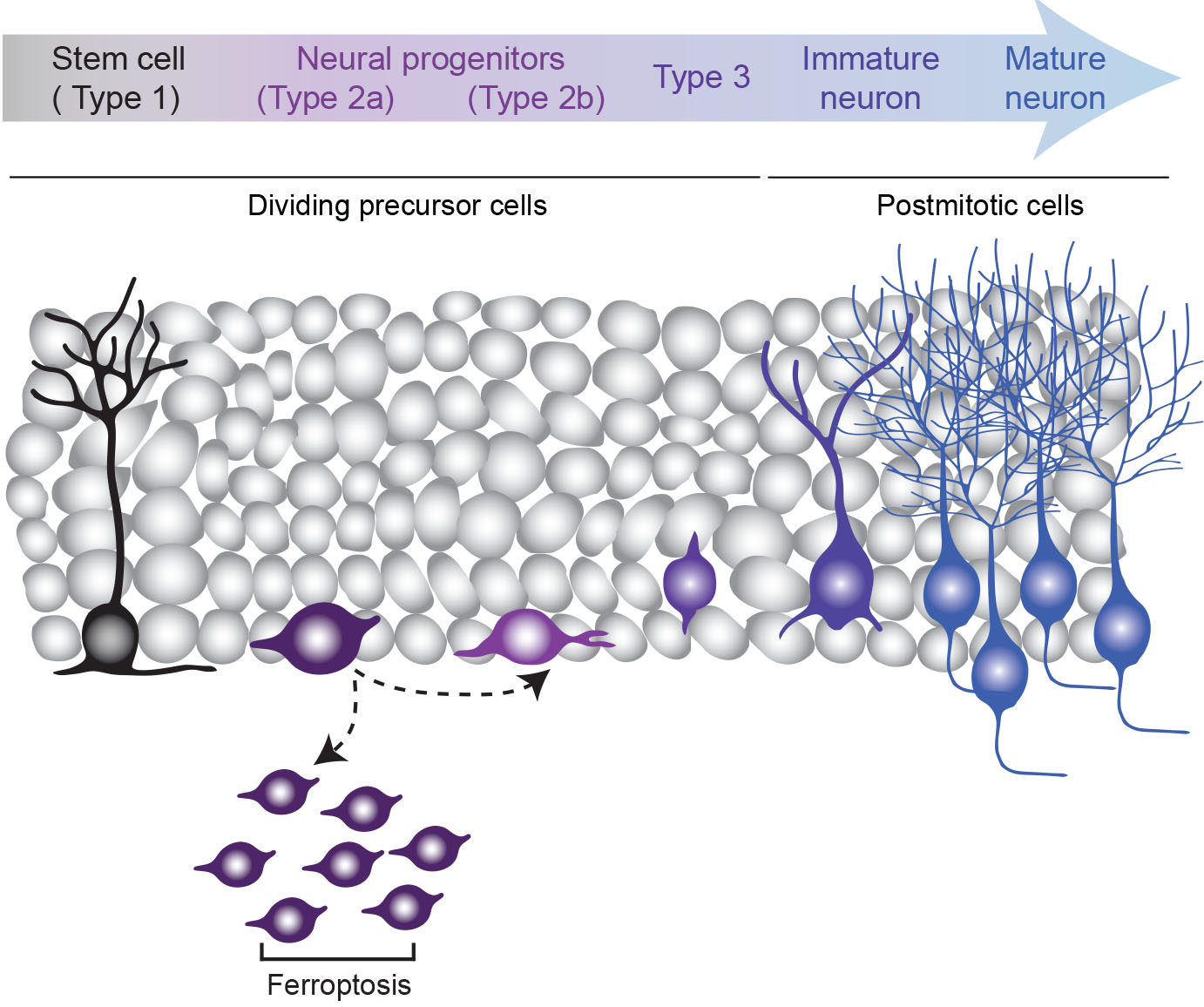
Adult neurogenesis is subject to refinement via regulated cell death, with more than 50% of the proliferating NPCs dying before they become mature integrated neurons. This was previously thought to only occur via apoptotic cell death. However, selenium is rate limiting for the production of the selenoprotein glutathione peroxidase 4 (Gpx4), which is the master regulator of a newly identified ferroptotic cell death pathway. Ferroptosis is a caspase-3-independent form of regulated cell death that is characterised by the iron-dependent accumulation of lethal reactive oxygen species (ROS) and lipid peroxidation products. Our research has provided the first evidence that selenium decreases the ferroptotic cell death of NPCs in the adult hippocampus. We therefore propose that selenium-mediated perturbations to the ferroptotic cell-death pathway explain the resulting increase in adult neurogenesis. The overall aim of this project is to determine whether selenium-mediated ferroptotic cell death is a fundamental mechanism underlying the regulation of adult hippocampal neurogenesis. This will provide significant benefit by identifying a novel cell death pathway that could be targeted to increase new neuron production.
Neurogenesis: schematic image of cells
Research approach (the initiative)
- To determine the involvement of selenium transport in ferroptotic cell death.
This study will reveal whether the trace element selenium has a novel role in mediating brain function. Specifically, these experiments will determine whether the interaction between the selenium transporter Sepp1 and its receptor Lrp8 at the blood brain barrier is required for selenium to enter the brain and protect NPCs from ferroptotic cell death, and whether this interaction underlies the exercise-induced increase in neurogenesis.
- To determine whether genetic perturbations to the ferroptosis pathway alter neurogenesis and associated cognitive function.
Using a series of complementary genetic approaches, these experiments will provide the first unequivocal evidence that NPCs are sensitive to ferroptosis and that this mode of cell death mediates the important process of adult hippocampal neurogenesis and associated cognitive function.
- To determine whether pharmacological inhibition of ferroptosis improves cognitive function in ageing and neurodegeneration.
These experiments will provide the first evidence as to whether inhibiting the ferroptotic cell death during physiological ageing and neurodegeneration (stroke, motor neuron disease and Alzheimer’s disease) can slow the decline in cognitive function.
Impacts and applications
The identification of the mechanism underlying the exercise-induced increase in adult neurogenesis will facilitate the discovery of novel therapeutic interventions (including ferroptosis inhibitors, such as liproxstatin-1, or dietary selenium supplementation) which can be used to target this pathway. The prospect of mimicking the beneficial effects of exercise on cognitive function using a simple dietary intervention such as selenium supplementation is extremely enticing. Given that selenium is a cheap, readily available dietary supplement that is found in a number of commonly eaten foods such as nuts, grains and dairy products, it could easily be boosted in the diet of elderly people. This is particularly important for the treatment of individuals who are unable to exercise due to advanced age, frailty or disability. Importantly, this work could also provide significant benefit in other cases of neuronal death, including stroke, brain injury, and neurodegenerative diseases, all conditions in which selenium-mediated ferroptotic cell death has been recently implicated.
Partners/collaborators
- Professor Ashely Bush
- Dr Scott Ayton
- Professor Gerd Kempermann
- Emeritus Professor Perry Bartlett
- Professor Shengtao Hou
- Dr Adam Walker


